
The practice of medicine and medical research is undergoing a rapid digital revolution transforming healthcare into a science of data analytics. The term Digital Health has entered the lexicon as we increasingly understand and describe medicine in terms of data generated by wearable physiological sensors, genetic sequencing and medical imaging. Rock Health, a venture fund dedicated to the founding and growth of Digital Health companies, recently published a report on the data driven transformation of healthcare, in particular highlighting the urgent need to develop digital biomarkers. Digital biomarkers are the medical signal or signatures contained in the vast data sets generated by the ever increasing use of leading edge medical technologies.
The Digital Health revolution has in large part been fueled by the growing adoption of wearable technologies. These include both FDA cleared medical sensors such as ECG patches as well as sensors marketed directly to consumers, for example, activity trackers from Apple, Fitbit, Garmin, Adidas and Misfit/Fossil. All classes of these wearable sensors are increasingly being incorporated into clinical research and healthcare delivery generating enormous repositories of medical data. This data is now being mined for new insights into drug and treatment effects in human health and well being. The Rock Health research continues with a cautionary quote and a call to innovation from Adam Jones, Assistant Vice President of Business Development at L’Oreal Research: “Collecting data is the ‘easiest’ part of the (Digital Health) process. Linking the data to some relevant phenotype is the hardest part—what clinical outcome can we predict and what can we associate with it?”
Clinically meaningful biomarkers may be identified by translating these mountains of digital data into informative and actionable medical insights. To reliably extract meaning from data in uncontrolled environments, it is critical to appropriately manage noise and meaningless signals that inevitably occur with wearable biosensors. When large volumes of data are scrubbed (noise removed), analyzed and summarized using appropriate and intelligent analytical software, such as the VivoSense® digital analysis platform, trends and patterns may be identified in unique patient populations. This allows subjective self-reported outcomes in psychological or neurological diseases to be studied with the addition of objective sensor data that directly relates to the severity of the disease state. The Vivonoetics team have decades of combined experience and are at the leading edge in managing artifact from wearable sensors and identifying digital biomarkers across therapeutic specialties.
The rapid adoption of wearable biosensors also presents a new opportunity to accelerate pharmaceutical/device clinical trials while driving down overall trial costs. Digital health sensors provide the opportunity to conduct large clinical trials in real world settings outside the traditional confinements of a medical setting. Sensors allow much more extensive, continuous data to be collected during trials and also allow cost control by reducing the need for patients to travel to clinical sites to interact with clinical personnel. At these visits, medical personnel may only be able to interact with the patient briefly collecting only limited, discrete data points.
Vivonoetics’ software tools that can reliably clean and analyze physiological data are critical in driving future discovery of new and meaningful digital biomarkers. We pride ourselves in providing a proven, validated sensor agnostic platform for digital biomarker identification and development. With these new tools we can realize the promise of clinical trial cost reduction while at the same time improve data quality to produce better medicines and ultimately achieve enhanced patient outcomes.
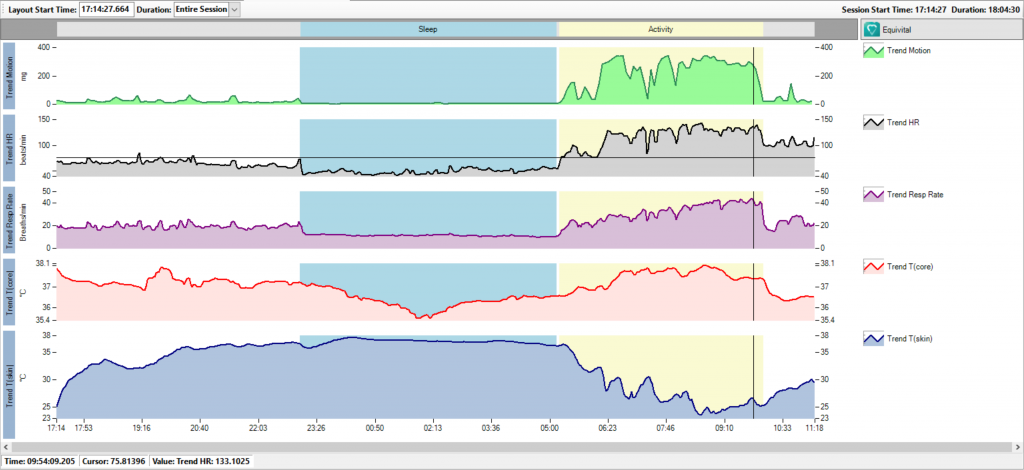
VivoSense® provides contextual analysis of digital biomarkers. The screenshot displays typical biomarkers (HR and Resp Rate) derived from continuous data and measured during periods of sleep and activity.
